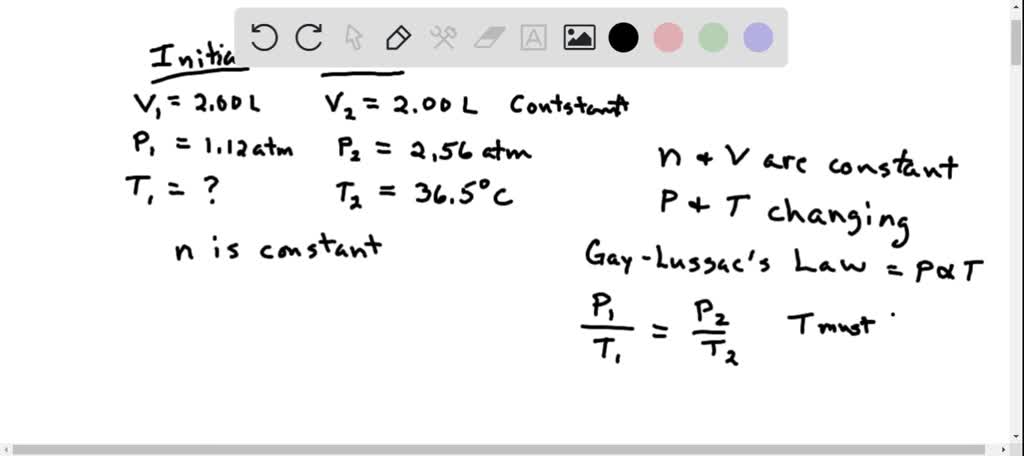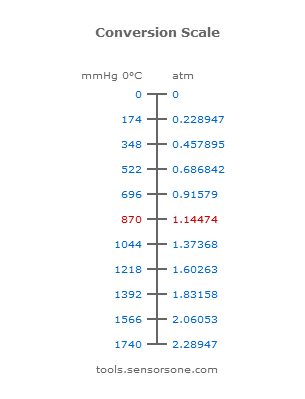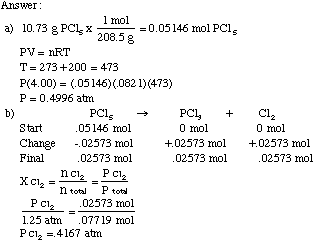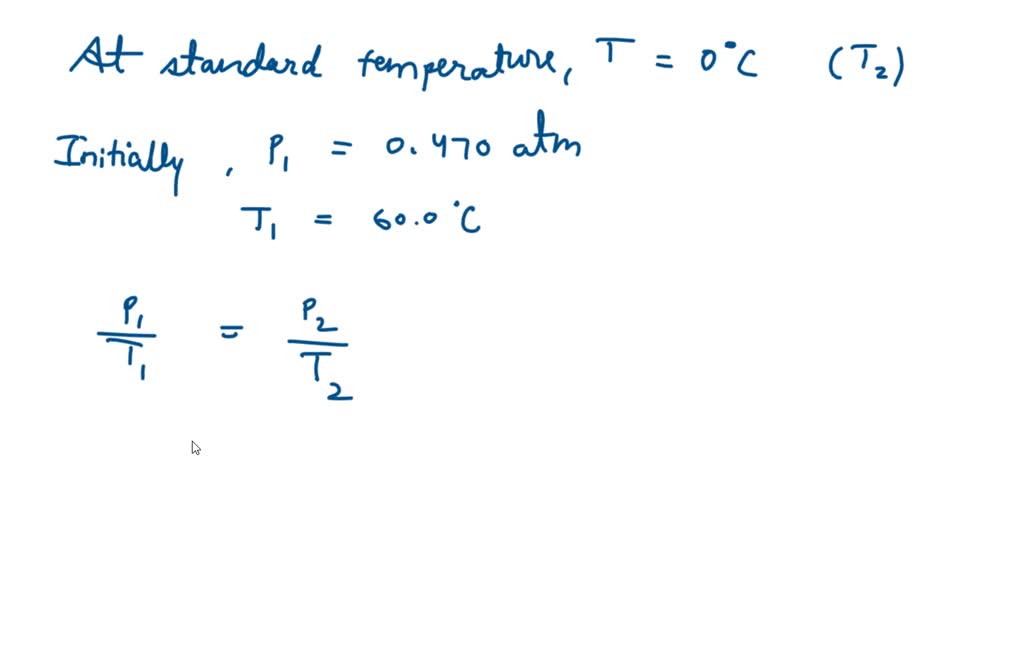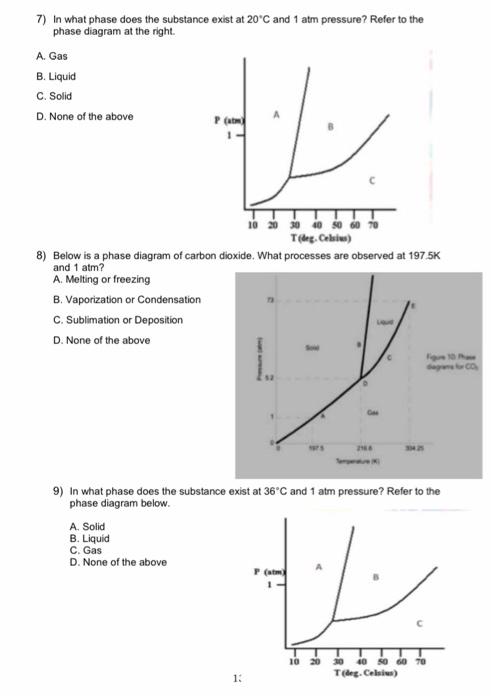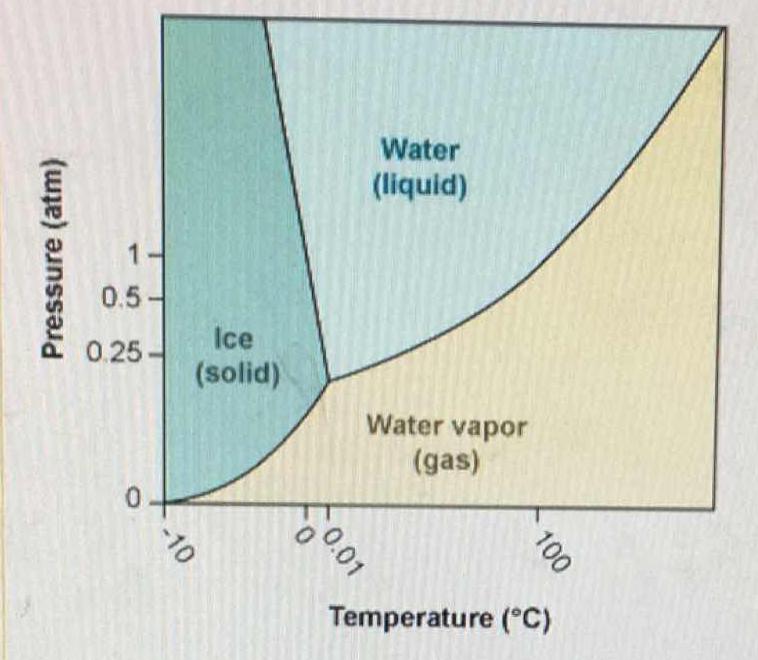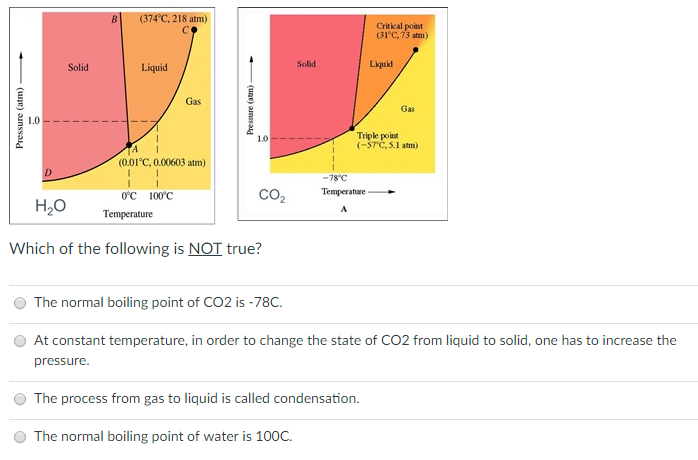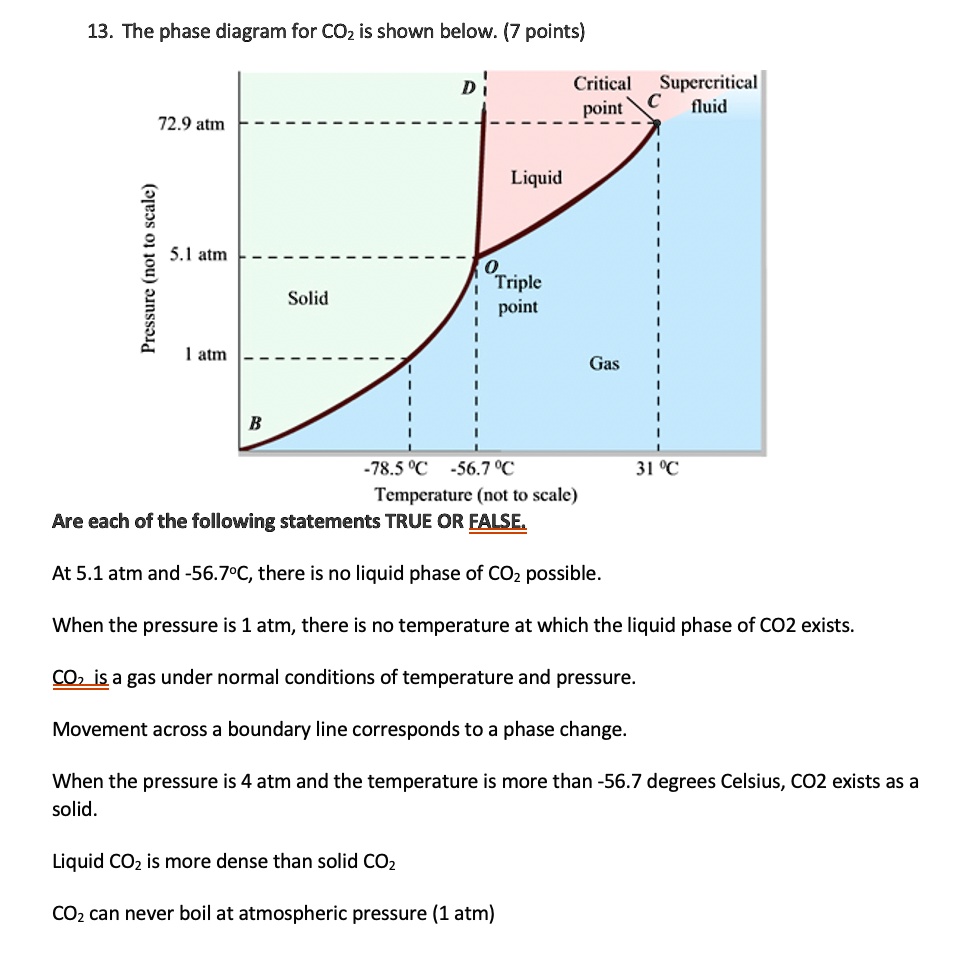
SOLVED: 13 The phase diagram for COz is shown below: points) Critical point Supereritical fluid 72.9 atm Liquid 1 1 5.1 atm L aln Triple point Solid Gas 78.5 %C 56.7 "€

Gas Pressure Unit Conversions - torr to atm, psi to atm, atm to mm Hg, kpa to mm Hg, psi to torr - YouTube

Gas Laws. A. Characteristics of Gases Gases expand to fill any container. –random motion, no attraction Gases are fluids (like liquids). –no attraction. - ppt download

At 546^oC and 1 atm pressure, 0.062 grams of gas occupies a volume of 33.6 ml . The molecular weight of the gas is: