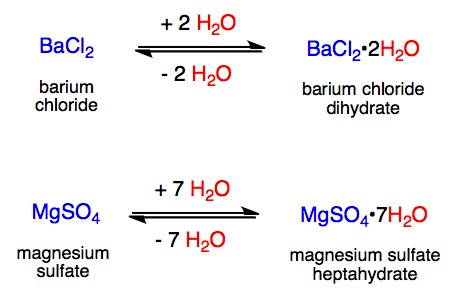
![SOLVED: the calculated masses (from IIl ] ) of anhydrous salt and water and the identity of the hydrated Use- compound (on the data slip supplied with the experiment) determine the number SOLVED: the calculated masses (from IIl ] ) of anhydrous salt and water and the identity of the hydrated Use- compound (on the data slip supplied with the experiment) determine the number](https://cdn.numerade.com/ask_images/8cd64c758f094cb482deaf5fb94b9dce.jpg)
SOLVED: the calculated masses (from IIl ] ) of anhydrous salt and water and the identity of the hydrated Use- compound (on the data slip supplied with the experiment) determine the number

5 g of crystalline salt, when rendered anhydrous, lost 1.89 g of water. The formula weight of anhydrous salts is 160 . The number of molecules of water of crystallisation in the salt is:

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol^-1 of heat. The value of ΔHydration AB is - 29.4 J mol^-1 . The heat of dissolution of

One mole of anhydrous salt AB dissolves in water and librates 21.0 J mol ^-1 of heat. The value of Δ H(hydration) of AB is - 29.4 J mol ^-1 . The
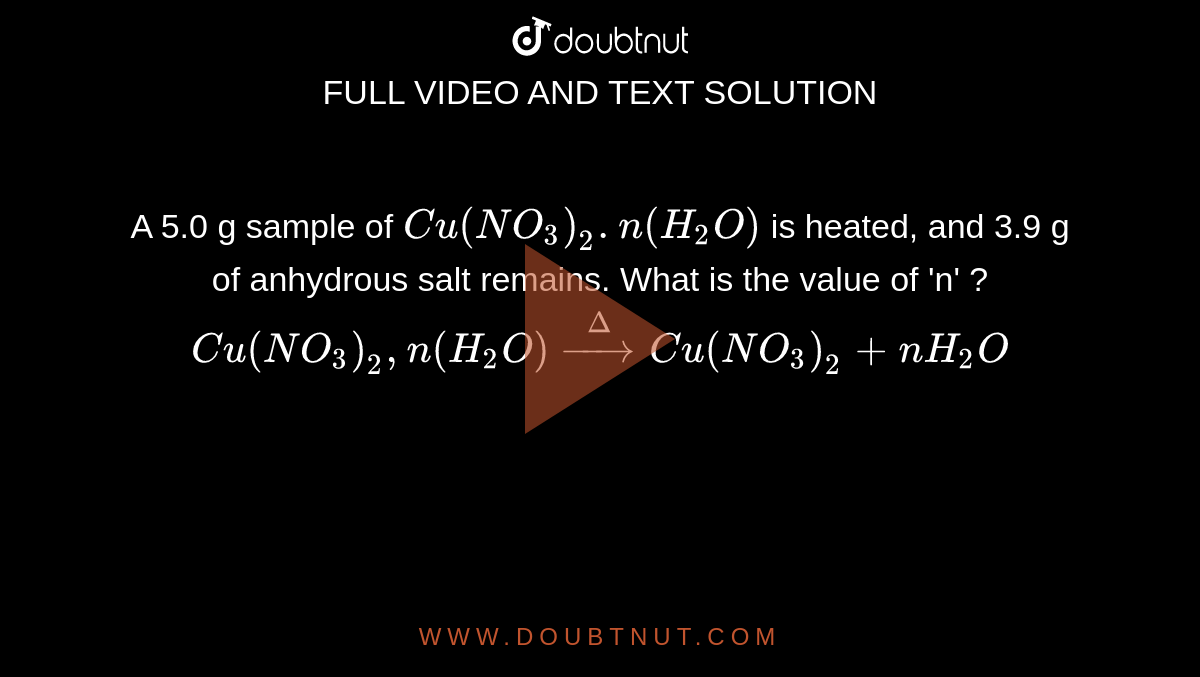
A 5.0 g sample of Cu (NO(3))(2).n (H(2)O) is heated, and 3.9 g of anhydrous salt remains. What is the value of 'n' ? Cu(NO(3))(2),n(H(2)O)overset(Delta)toCu(NO(3))(2)+nH(2)O
One mole of anhydrous salt AB dissolves inwater and liberates 15 J mol 1 of heat. The value ofdrs fon of AB is 20.5 J mol 1. Hence, the enthalpyof dissolution of

SOLVED: Formula (Molar) mass of anhydrous salt (on bottle) Number of grams of HzO per 100.0 g hydrate *49 14.8 Number of moles HzO per 100.0 g hydrate 1q.8 18.0 Da moles

A treatise on the theory of solution including the phenomena of electrolysis . °6 and pass into the anhydrous salt andwater. Another hydrate, Na2S04. 7 HgO can be obtained byadding alcohol to
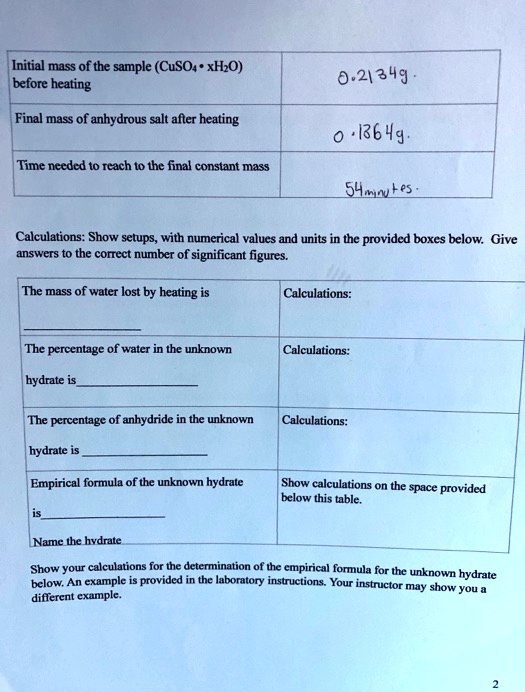
SOLVED: Initial mass of the sample (CuSO4 * xHO) before beating 0.21349 Final mass of anhydrous salt after heating Time needed t0 reach t0 the final constant mass S41uLes Calculations: Show setups;




.PNG)